The future of personalized medicine: Technion team built blood tree from scratch
Currently, transplanted grafts need to be implanted into a healthy part of the body so that the patient can generate new blood vessels to support it.
By HADAS LABRISCH , Jerusalem Post, SEPTEMBER 20, 2021
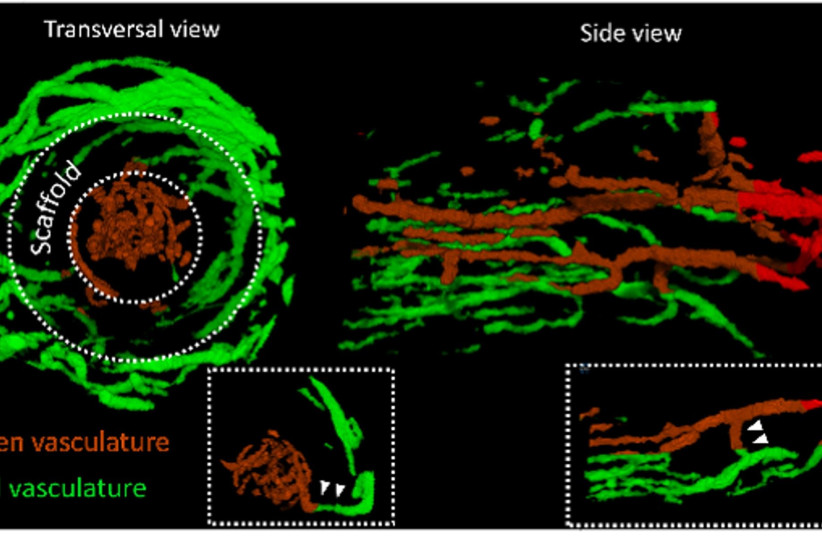
Engineered blood vessels in Technion study.
Vascular structures in the scaffold lumen (brown) communicate with
vessels located in the surrounding hydrogel (green). (photo credit: Courtesy)
Skin flaps, bone grafts, implanted tissue - recent advancements in
medicine have changed the face of surgery in terms of autologous -
meaning self - transplantations.
While
extensive damage to organs once meant a nearly sure amputation or need
for an external transplant, today's science focuses on harvesting cells
and tissue from a person's own body to complete the injured pieces of
the puzzle, using grafts and flaps to repair skin, vessels, tubes and
bones.
Yet, ask
any surgeon attempting to insert a flap and they would tell you that the
most important - and restrictive - component of a graft's success is
ample blood supply.
A team of researchers at the Technion
recently found a way to meet this need. For the first time, these
scientists succeeded in 3D printing a network of big and small blood
vessels that could provide blood to implanted tissues just like the
human body.
Up
until now, medicine hasn't been able to mimic the body's ability to
create a suitable hierarchy in the blood vessel tree. In our bodies, the
heart pumps blood into a large tube called the aorta, which measures
roughly 2-3 cm in diameter. The blood vessels then branch off into
smaller and smaller tubes that are appropriate to each organ's need and
capacity, until they reach minuscule arterioles of only 5 to 10
micrometers.
HUMAN BODY circulatory system showing the heart and blood vessels (credit: FLICKR)
Dr.
Ariel Alejandro Szklanny of the Technion team, led by Professor
Shulamit Levenberg, a specialist in tissue engineering, found a way to
use 3D printing to form a system containing a functional combination of both the large and small vessels.
The
new breakthrough may allow a tissue flap to be created in a lab already
connected to a blood network suited to its size and function.
Currently,
transplanted grafts need to be implanted into a healthy part of the
body so that the patient can generate new blood vessels to support it;
then, the graft is relocated to an affected area as healthy tissue.
The new technique could potentially eradicate this intermediate
step, drastically improving recovery times and cutting down on the
number of procedures a patient would need to undergo.
In his recently published study in Advanced Materials,
Dr. Szklanny described how he created a polymeric scaffold filled with
small holes, mimicking the large blood vessels of the body. These holes
allowed the connection of smaller vessels to join into the engineered
large vessels. With collagen bio-ink, the team then printed and
assembled a complex network around and within the main scaffold, later
covering it with endothelial (human blood vessel lining) cells. A week
later, the incubated artificial apparatus joined with the cells to
create a hierarchical structure just like the human blood vessel tree.
Levenberg–Szklanny Advanced Materials 6, August 23, 2021
While previous studies in this field used animal-borne collagen,
the Technion team used engineered tobacco plants created by the Israeli
company CollPlant.
The
mesh was transplanted into a study rat and attached to the main artery
in its leg. The blood through the artery spread through the network
exactly as it would within the body, carrying oxygen and nutrients to
the distant parts of the implanted tissue, and without any leaks.
This
achievement is an important tool in the world of personalized medicine
and could be a huge leap forward in tissue engineering and treatment.
PLEASE RECOMMEND THIS PAGE AND FOLLOW US AT:

No comments:
Post a Comment
Stick to the subject, NO religion, or Party politics