The herb rosemary has long been associated with memory: “There’s rosemary, that’s for remembrance,” says Ophelia in Shakespeare’s Hamlet. Fittingly, researchers have studied a compound found in rosemary and sage—carnosic acid—for its potential impact on Alzheimer’s disease. This disease, the leading cause of dementia and the sixth leading cause of death in the U.S., is often accompanied by inflammation, which contributes to cognitive decline.
Carnosic acid is an antioxidant and anti-inflammatory compound that activates enzymes forming the body’s natural defense system. However, pure carnosic acid is too unstable for use as a drug. To address this, scientists at Scripps Research have synthesized a stable form called diAcCA, which is fully converted to carnosic acid in the gut before being absorbed into the bloodstream.
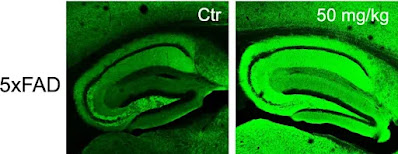
The research, published in Antioxidants on February 28, 2025, showed that when diAcCA was used to treat mouse models of Alzheimer’s disease, it achieved therapeutic doses of carnosic acid in the brain and led to enhanced memory and synaptic density, or more synapses (representing the connections between nerve cells), in the brain. Because the decline of neuronal synapses is also closely correlated to dementia in Alzheimer’s disease, this approach could counteract the progression of cognitive decline.
Reducing Brain Inflammation and Enhancing Synaptic Density
Analysis of tissue samples showed the drug also markedly decreased inflammation in the brain. This unique drug is activated by the very inflammation that it then combats and thus is only active in areas of the brain undergoing inflammatory damage. This selectivity limits the potential side effects of carnosic acid, which is on the US Food and Drug Administration’s “generally regarded as safe” (GRAS) list, easing the way for clinical trials.
“By combating inflammation and oxidative stress with this diAcCA compound, we actually increased the number of synapses in the brain,” says senior author and professor Stuart Lipton, MD, PhD, the Step Family Foundation Endowed Chair at Scripps Research and a clinical neurologist in La Jolla, California. “We also took down other misfolded or aggregated proteins such as phosphorylated-tau and amyloid-β, which are thought to trigger Alzheimer’s disease and serve as biomarkers of the disease process.”
Lipton’s group had previously determined that carnosic acid crosses the blood-brain barrier and activates the Nrf2 transcriptional pathway, which turns on antioxidant and anti-inflammatory genes. But the compound oxidizes easily, making it unsuitable as a drug because of its short shelf-life.
In this new study, Lipton and co-author Phil Baran, PhD, the Dr. Richard A. Lerner Endowed Chair in the Department of Chemistry at Scripps Research, synthesized a range of carnosic acid derivatives and selected diAcCA as the best candidate because of its stability, bioavailability, and other drug-like properties. Lipton’s group then treated mouse models with the compound over the course of three months. The group examined the mice by testing their spatial learning and memory in behavioral tests and then analyzing brain tissue under the microscope.
“We did multiple different tests of memory, and they were all improved with the drug,” Lipton says. “And it didn’t just slow down the decline; it improved virtually back to normal.” Analysis of tissues also showed increased neuronal synaptic density and decreased formation of phosphorylated-tau aggregates and amyloid-β plaques.
Safety and Potential Applications
The mice tolerated diAcCA well. In toxicity studies, the compound even soothed baseline inflammation in the esophagus and stomach as it was converted to carnosic acid.
The group also found that the mice took up about 20% more carnosic acid after ingesting diAcCA than they did after taking plain carnosic acid. Because most carnosic acid oxidizes while being stored or upon ingestion, “diAcCA produces more carnosic acid in the blood than if you took carnosic acid itself,” Lipton explains.
Lipton sees a potential for diAcCA to work in tandem with Alzheimer’s treatments currently on the market. Not only could the drug work on its own by combating inflammation, but “it could make existing amyloid antibody treatments work better by taking away or limiting their side effects” such as a form of brain swelling or bleeding known as ARIA-E and ARIA-H, he says.
Lipton hopes diAcCA can be fast-tracked through clinical trials because of its safety profile. He thinks it could also be explored as a treatment for other disorders marked by inflammation, such as type 2 diabetes, heart disease, and other forms of neurodegeneration such as Parkinson’s disease.
The Life of Earth


No comments:
Post a Comment
Stick to the subject, NO religion, or Party politics